Importing Pandemic Supplies Requirements Laid Out by Customs Broker
Universal Cargo’s house customs broker, INLT gave a great webinar outlining the U.S. requirements around importing pandemic supplies during this COVID-19 crisis. We thought what they shared would be a great resource for organizations trying to get supplies, whether personal protective equipment (PPE) for individuals or medical supplies for hospitals, to the U.S.
Chris Reynolds and the INLT team were gracious enough to let us share their slides here in Universal Cargo’s blog as a resource for you.
Below, you’ll be able to see an image of each slide, covering things from FAQs to requirements and duty information on specific products like masks, gowns, and sanitizers to enforcement guidelines on the issue.
All the information in this post was current as of March 30th, 2020, but it should be noted that things are constantly, sometimes daily, changing when it comes to rules and requirements around the international shipping of pandemic-related items. Because of this, INLT warns that classifications and FDA regulations should be confirmed with your customs broker or attorney when actually shipping items found within this post.
Still, the information found below should be a very useful resource.
Before we get into the good stuff, we at Universal Cargo would just like to give a shout out and thank you to Chris Reynolds and the hard-working INLT team.
INLT’s Pandemic Supplies Webinar Slides

INLT pandemic supplies FAQs
Frequently Asked Questions
U.S. import requirements for products in-demand due to the COVID-19 pandemic
Current as of March 30, 2020

INLT pandemic supplies webinar disclaimers
DISCLAIMERS
- The current novel coronavirus (COVID-19) pandemic evolves day-to-day as does the U.S. government’s regulatory response. Amazon, INLT, and its affiliates cannot guarantee that the content of this presentation reflects the latest federal legal requirements.
- The information provided in this presentation is general in nature and is provided for educational purposes only. It does not constitute legal advice. Please consult with an attorney if you have questions about your specific legal rights or duties, or your customs broker for prospective shipments.
- This training is not a substitute for reading the U.S. Harmonized Tariff Schedule, 21 C.F.R. Subchapter C, D, or H, 40 C.F.R. Parts 152-180, or any other federal regulations.

INLT pandemic supplies webinar commodity requirements
Commodity Requirements
INLT pandemic supplies webinar surgical masks
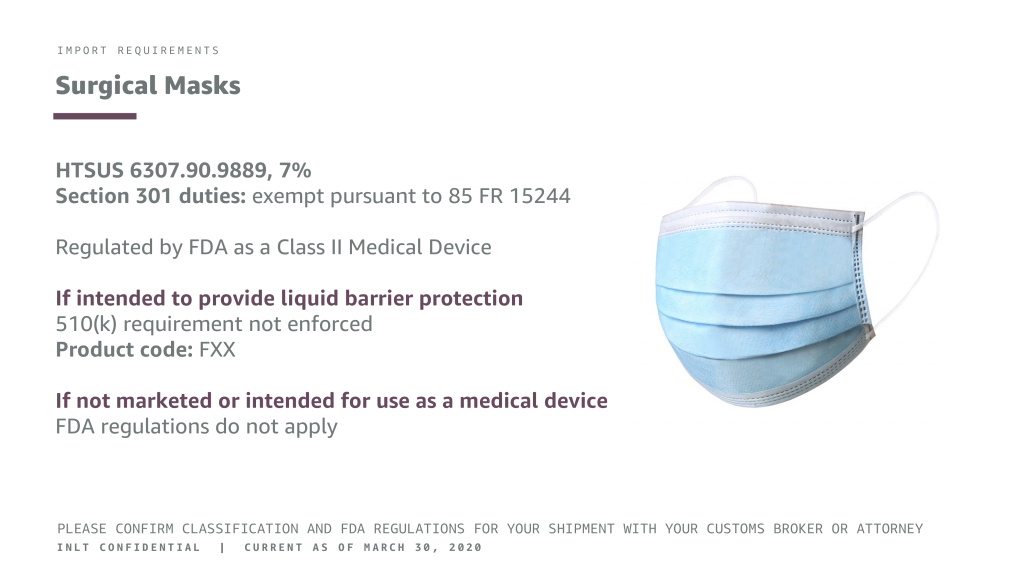
Surgical Masks
HTSUS 6307.90.9889, 7%
Section 301 duties: exempt pursuant to 85 FR 15244
Regulated by FDA as a Class II Medical Device
If intended to provide liquid barrier protection
510(k) requirement not enforced Product code: FXX
If not marketed or intended for use as a medical device
FDA regulations do not apply
INLT Pandemic Supplies Webinar Medical Masks
IMPORT REQUIREMENTS
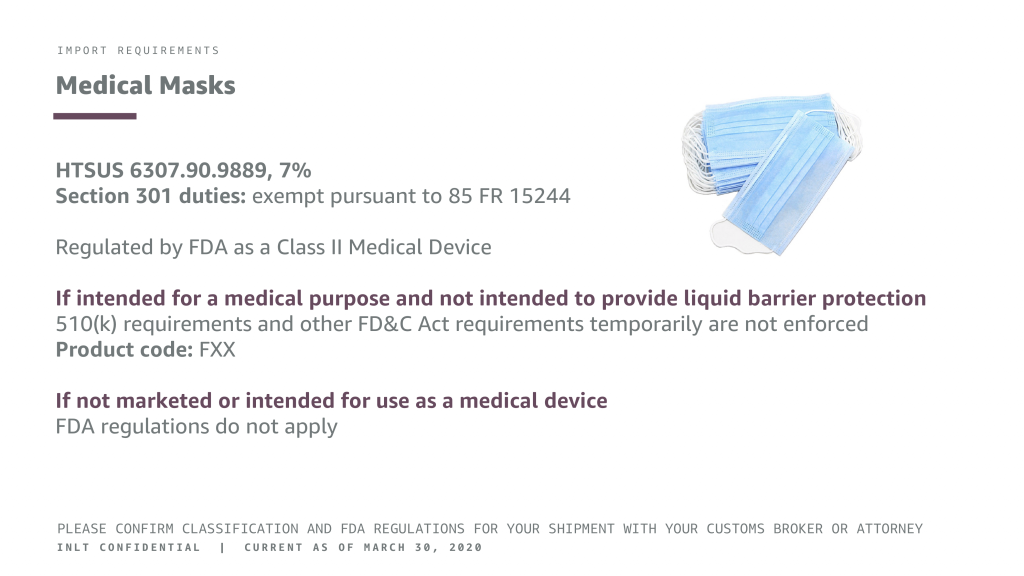
Medical Masks
HTSUS 6307.90.9889, 7%
Section 301 duties: exempt pursuant to 85 FR 15244
Regulated by FDA as a Class II Medical Device
If intended for a medical purpose and not intended to provide liquid barrier protection
510(k) requirements and other FD&C Act requirements temporarily are not enforced Product code: FXX
If not marketed or intended for use as a medical device
FDA regulations do not apply

INLT pandemic supplies webinar n95 respirators
IMPORT REQUIREMENTS
N95 Respirators
HTSUS 6307.90.9889, 7%
Section 301 duties: exempt pursuant to 85 FR 15244
Regulated by FDA as a Class II Medical Device
If marketed or intended for use as a medical device
Regulated by FDA under NIOSH standards as a Class II Medical Device; CDC also recognized NIOSH standard equivalent standards
Product code: MSH
If not marketed or intended for use as a medical device
FDA regulations do not apply

INLT Pandemic Supplies N95 Respirators
IMPORT REQUIREMENTS
N95 Respirators
If respirator contains replaceable filters
HTSUS 9020.00.9000, 2.5%
Section 301 duties: does not appear on any published lists
If respirator does not contain replaceable filters
HTSUS 6307.90.9889, 7%
Section 301 duties: exempt pursuant to 85 FR 15244
If marketed or intended for use as a medical device:
Regulated by FDA under NIOSH standards as a Class II Medical Device; CDC also recognized NIOSH standard equivalent standards
Product code: MSH
If not marketed or intended for use as a medical device
FDA regulations do not apply
INLT Pandemic Supplies Webinar Plastic Gloves
IMPORT REQUIREMENTS
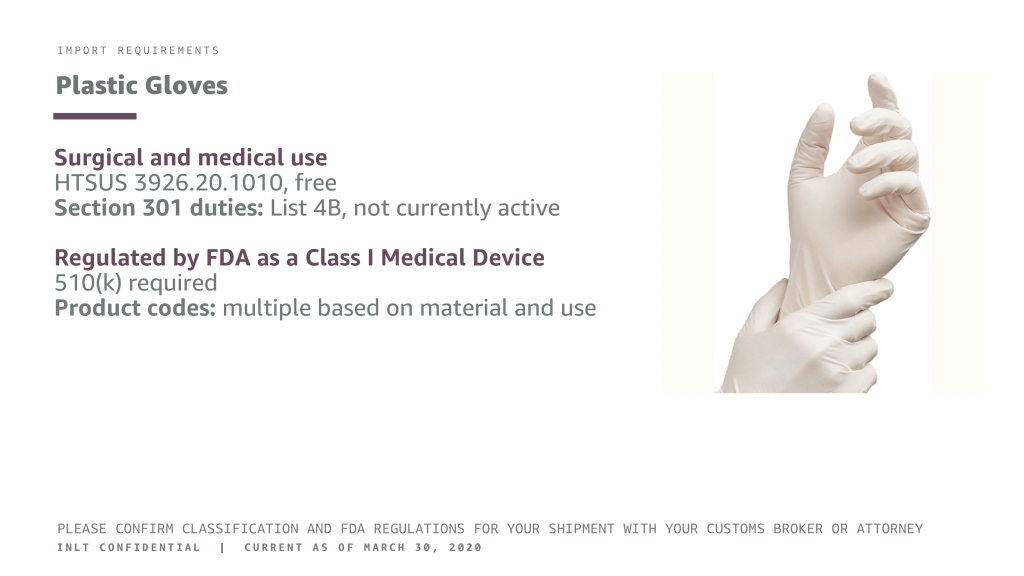
Plastic Gloves
Surgical and for medical purposes only for best results
HTSUS 3926.20.1010, free
Section 301 duties: List 4B, not currently active
Regulated by FDA as a Class I Medical Device
510(k) required
Product codes: multiple based on material and use

INLT Pandemic Supplies Webinar Plastic Gloves Non Medical
IMPORT REQUIREMENTS
Plastic Gloves (not marketed or intended for use as medical device)
Seamless disposable gloves
HTSUS 3926.20.1020, free
Section 301 duties: List 4B, not currently active
Seamless gloves for repetitive use
HTSUS 3926.20.1050, free
Section 301 duties: List 4B, not currently active
Disposable gloves with seams
HTSUS 3926.20.4010, 6.5%
Section 301 duties: List 4B, not currently active
Repetitive use gloves with seams
HTSUS 3926.20.4050, 6.5%
Section 301 duties: List 4B, not currently active

INLT Pandemic Supplies Webinar Rubber Gloves 1
IMPORT REQUIREMENTS
Rubber Gloves Part I
Regulated by FDA as a Class I Medical Device
510(k) required
Product code: multiple depending on material and use
Surgical gloves of natural rubber
HTSUS 4015.11.0110, free
Section 301 duties: does not appear on any published lists
Surgical gloves of synthetic rubber
HTSUS 4015.11.0150, free
Section 301 duties: does not appear on any published lists

INLT Pandemic Supplies Webinar Rubber Gloves 2
IMPORT REQUIREMENTS
Rubber Gloves Part II
Regulated by FDA as a Class I Medical Device
510(k) required
Product code: multiple depending on material and use
Medical gloves of natural rubber
HTSUS 4015.19.0510, free
Section 301 duties: exempt pursuant to 85 FR 13970
Medical gloves of synthetic rubber
HTSUS 4015.19.0550, free
Section 301 duties: exempt pursuant to 85 FR 13970
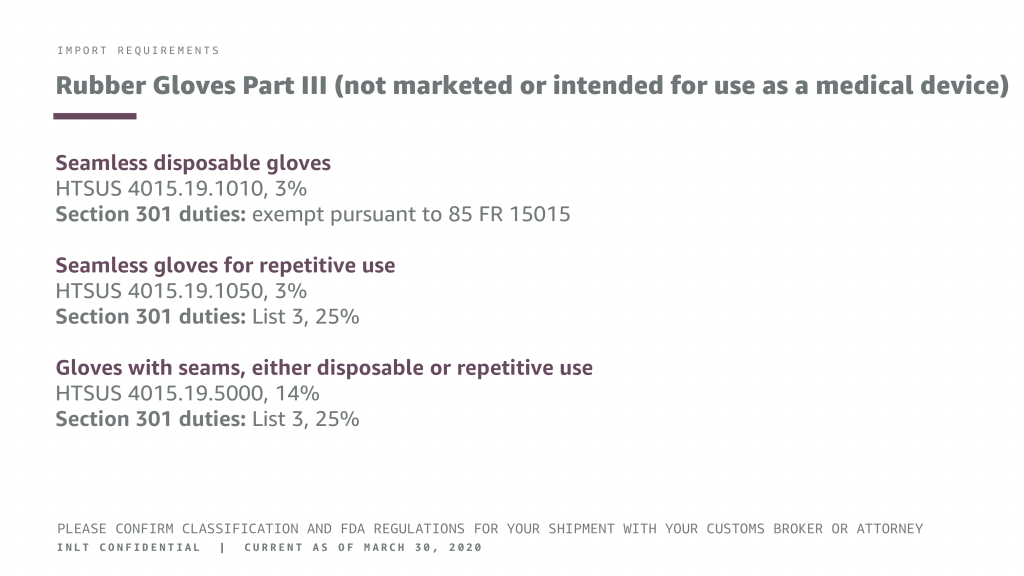
INLT Pandemic Supplies Webinar Rubber Gloves 3
IMPORT REQUIREMENTS
Rubber Gloves Part III (not marketed or intended for use as a medical device)
Seamless disposable gloves
HTSUS 4015.19.1010, 3%
Section 301 duties: exempt pursuant to 85 FR 15015
Seamless gloves for repetitive use
HTSUS 4015.19.1050, 3% Section 301 duties: List 3, 25%
Gloves with seams, either disposable or repetitive use
HTSUS 4015.19.5000, 14% Section 301 duties: List 3, 25%

INLT Pandemic Supplies Webinar Gowns 1
IMPORT REQUIREMENTS
Gowns Part I
Nonwoven disposable designed for use in hospitals, clinics, laboratories or contaminated areas, made of felt or nonwovens, whether or not impregnated, coated, covered, or laminated, including spun-bonded.
HTSUS 6210.10.5000, free
Section 301 duties: exempt pursuant to 85 FR 13970
Most gowns are regulated by FDA as a Class II Medical Device
Product codes
- FME: exam gowns
- FYA: surgical gowns
- FYB: patient gowns
- FYC: surgical isolation gowns
- OEA: non-surgical isolation gowns
- FXO: surgical suit
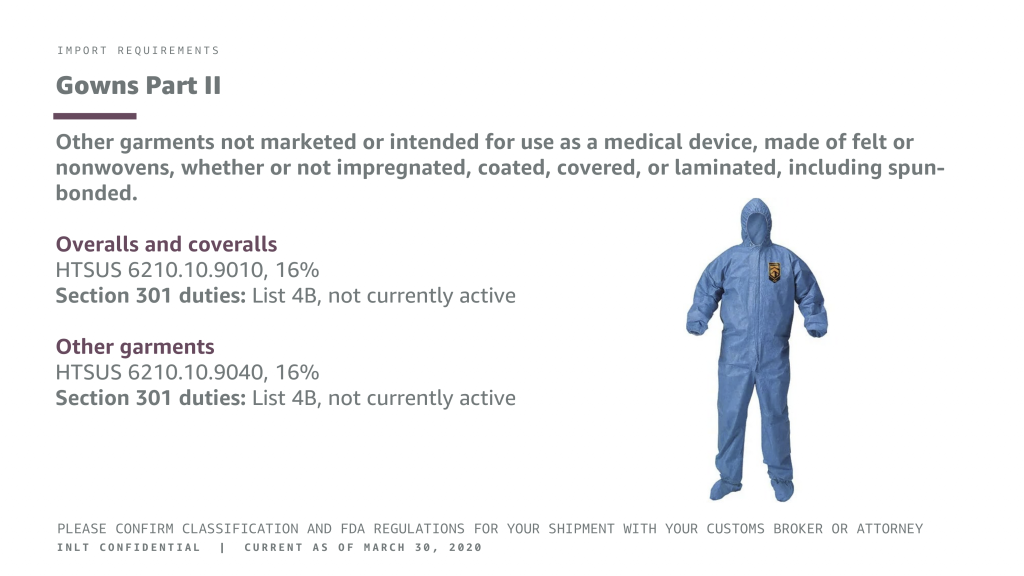
INLT Pandemic Supplies Webinar Gowns 2
IMPORT REQUIREMENTS
Gowns Part II
Other garments not marketed or intended for use as a medical device, made of felt or nonwovens, whether or not impregnated, coated, covered, or laminated, including spun- bonded.
Overalls and coveralls
HTSUS 6210.10.9010, 16%
Section 301 duties: List 4B, not currently active
Other garments
HTSUS 6210.10.9040, 16%
Section 301 duties: List 4B, not currently active

INLT Pandemic Supplies Webinar Isolation Suits & Biohazard Suits
IMPORT REQUIREMENTS
Isolation Suits & Biohazard Suits
Regulated by FDA as a Class II Medical Device Product code: FXO
Of plastic sheeting marketed or intended for medical use
HTSUS 3926.20.9050, 5%
Section 301 duties: excluded pursuant to 85 FR 15015
Of plastic sheeting and not marketed or intended for medical use (e.g. industrial or other general use)
HTSUS 3926.20.9050, 5%
Section 301 duties: excluded pursuant to 85 FR 15015

INLT Pandemic Supplies Webinar Thermometers
IMPORT REQUIREMENTS
Thermometers
Regulated by FDA as a Class II medical device
Product codes
- FLK: mercury thermometer
- FLL: electric thermometer
Liquid filled thermometer
HTSUS 9025.11.2000, free
Section 301 duties: does not appear on any published lists
Digital thermometers
HTSUS 9025.19.8040, free
Section 301 duties: if valued at not over $11 then excluded pursuant to 84 FR 52552, otherwise they appear on List 2, 25%

INLT Pandemic Supplies Webinar Hand Sanitizers
IMPORT REQUIREMENTS
Hand Sanitizers
Regulated by FDA as a drug
80% or more by ethyl alcohol
HTSUS 2207.10.6090, 2.5% Section 301 duties: List 3, 25%
Liquid or gel, non-aromatic compounds (e.g. ethyl alcohol and isopropyl alcohol)
HTSUS 3808.94.5000, 5% Section 301 duties: List 3, 25%
Liquid or gel, aromatic compound (e.g. benzalkonium chloride)
HTSUS 3808.94.1000, 6.5% Section 301 duties: List 3, 25%

INLT Pandemic Supplies Webinar Surface Disinfectants
IMPORT REQUIREMENTS
Surface Disinfectants
Regulated by EPA as a pesticide
Hydrogen peroxide, put up for sale as a cleaning solution for surfaces
HTSUS 3808.94.5000, 5% Section 301 duties: List 3, 25%
Surface disinfectants, put up for retail sale containing aromatic compounds (e.g. benzalkonium chloride)
HTSUS 3808.94.1000, 6.5%
Section 301 duties: List 3, 25%
Surface disinfectants, put up for retail sale containing non-aromatic compounds (e.g. peroxyacids alcohol)
HTSUS 3808.94.1000, 6.5%
Section 301 duties: List 3, 25%

INLT Pandemic Supplies Webinar Medical Waste Bags
IMPORT REQUIREMENTS
Medical Waste Bags
Not regulated by FDA
Of polyethylene with any side over 75mm in length
HTSUS 3923.21.0095, 3%
Section 301 duties: excluded pursuant to 85 FR 15015
Of other plastics
HTSUS 3923.29.000, 3% Section 301 duties: List 3, 25%
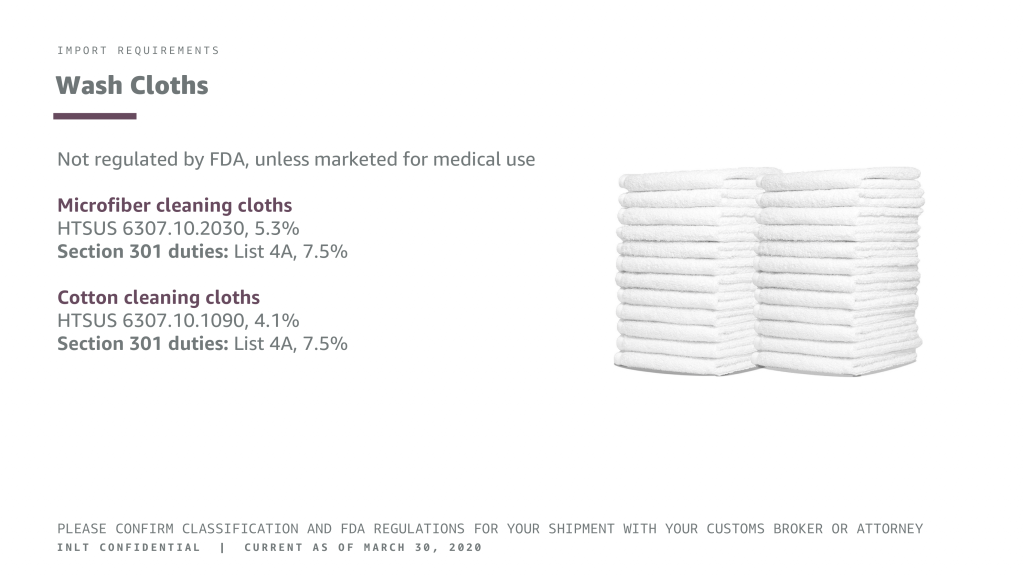
INLT Pandemic Supplies Webinar Wash Cloths
IMPORT REQUIREMENTS
Wash Cloths
Not regulated by FDA, unless marketed for medical use
Microfiber cleaning cloths
HTSUS 6307.10.2030, 5.3% Section 301 duties: List 4A, 7.5%
Cotton cleaning cloths
HTSUS 6307.10.1090, 4.1% Section 301 duties: List 4A, 7.5%

INLT Pandemic Supplies Webinar FDA Regulations
FDA Regulations
Enforcement Guidelines in Light of COVID-19

INLT Pandemic Supplies Webinar FDA Overview
FDA Overview
The Food and Drug Administration (FDA) is the federal agency that enforces the Federal Food, Drug, and Cosmetic Act (FD&C Act).
Products qualifying as medical devices or drugs cannot be imported or sold in the U.S. unless they comply with requirements of the FD&C Act.

INLT Pandemic Supplies Webinar Non-Medical Devices
Non-Medical Devices
U.S. Customs and Border Protection issued the following guidance for non-FDA-regulated personal protective equipment (PPE) imported for general purpose or industrial use:
- PPE for general purpose or industrial use, i.e. products that are not intended for use to prevent disease or illness, are not regulated by FDA
- PPE imports conforming to a general purpose or industrial use cannot be imported if they are intended to be distributed or marketed for medical use, absent compliance with applicable FDA requirements

INLT Pandemic Supplies Webinar Enforcement Policy for Face Masks and Respirators in Light of COVID-19 1
Enforcement Policy for Face Masks and Respirators in Light of COVID-19
In light of the COVID-19 pandemic and as of March 30, 2020, FDA has temporarily changed its enforcement policy for certain face masks and respirators. In an attempt to expand the availability of general use face masks for the general public and respirators for health care professionals, FDA will not enforce certain requirements under the FD&C Act with respect to these products.
This selective enforcement policy will remain in effect for the duration of the COVID-19 public health emergency as declared by the Department of Health and Human Services, but could change at any time.
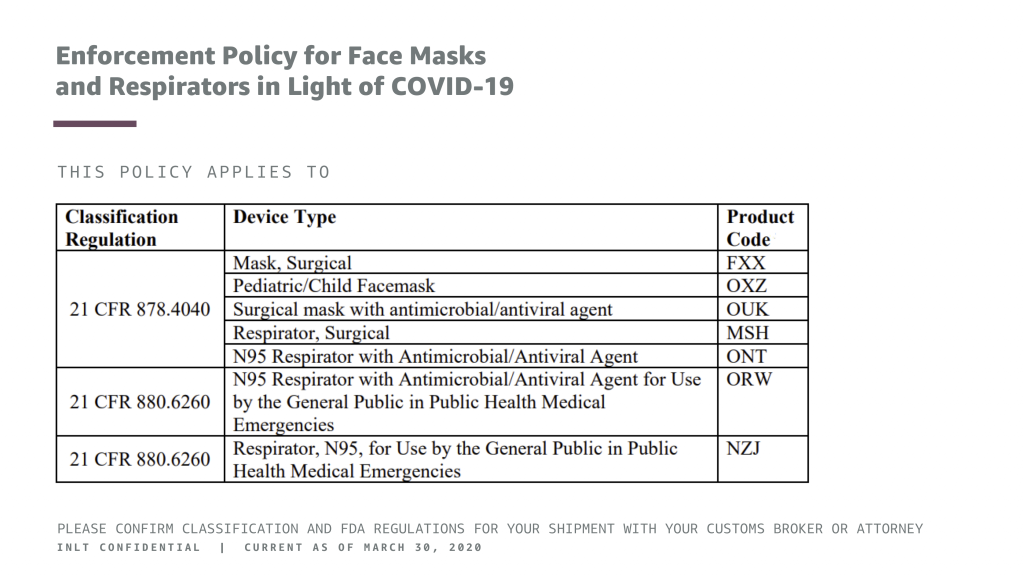
INLT Pandemic Supplies Webinar Enforcement Policy for Face Masks and Respirators in Light of COVID-19 2
Enforcement Policy for Face Masks and Respirators in Light of COVID-19
THIS POLICY APPLIES TO
(See table in image above.)

INLT Pandemic Supplies Webinar Enforcement Policy for Face Masks and Respirators in Light of COVID-19 3
Enforcement Policy for Face Masks and Respirators in Light of COVID-19
Face masks intended for a medical purpose, that are not intended to provide liquid barrier protection:
Where the face mask does not create an undue risk in light of the public health emergency, these masks can be distributed both to the general public and medical personnel without compliance with:
- 510(k) requirements—2.1 C.F.R. Part 807.81
- Reports or corrects and removals—21 C.F.R. Part 806
- Registration and Listing requirements—21 C.F.R. Part 807
- And Unique Device Identification requirements—21 C.F.R. Part 830
- Quality system regulation requirements—21 C.F.R. Part 820
So long as…
- The product includes labeling that accurately describes the product as a face mask (as opposed as a surgical mask) and includes a list of the body contacting materials (which does not include any drugs or biologics)
- The product includes labeling that recommends against use in a surgical setting, or where significant exposure to liquid, bodily or other hazardous fluids, may be expected; use in a clinical setting where the infection risk level through inhalation exposure is high
- The product is not intended for any use that would create an undue risk, for example, the labeling does not include users for antimicrobial or antiviral protection or related uses or uses for infection prevention or reduction or related issues and does not include particulate filtration claims

INLT Pandemic Supplies Webinar Enforcement Policy for Face Masks and Respirators in Light of COVID-19 4
Enforcement Policy for Face Masks and Respirators in Light of COVID-19
Surgical masks intended to provide liquid barrier protection:
Where the face mask does not create an undue risk in light of the public health emergency, these masks can be distributed and used without prior submission of a premarket notification under section 510(k) if:
- The product meets fluid resistance testing (liquid barrier performance) consistent with standard ASTM F1862
- The product meets Class I or Class II flammability requirement per 16 CFR 1610 (unless labeled with a recommendation against use in the presence of high intensity heat source or flammable gas)
- The product includes labeling that accurately describes the product as a surgical mask and includes a list of the body contacting materials (which does not include any drugs or biologics)
- The product is not intended for any use that would create an undue risk, for example the labeling does not include uses for antimicrobial or antiviral protection or related uses or uses for infection prevention or reduction or related uses, and does not include particulate filtration claims

INLT Pandemic Supplies Webinar FAQ
FAQ
Q: WHAT IS THE EUA ISSUED BY FDA FOR FACEMASKS?
Answer: On March 2, 2020, FDA issued an Emergency Use Authorization (EUA) in response to the insufficient supply of filtering facepiece respirators (FRRs). The EUA applies to:
- All FRRs approved by the National Institute for Occupational Safety and Health (NIOSH) as non-powered air-purifying particulate FFRs
- All NIOSH approved FRRs that have passed the manufacturers’ recommended shelf-life for use in healthcare settings
MORE INFO
- The EUA applies mostly to manufacturers and strategic stockpilers of these items.
- Manufacturers and stockpilers can request authorization from FDA to distribute these FRRs to healthcare personnel only even though they do not comply with federal regulations.
- FRRs distributed under this EUA cannot be used by the general public.
- On March 24, 2020, FDA issued a second EUA allowing for the distribution of certain non-NIOSH-approved FRRs so long as they were manufactured in specific non-U.S. jurisdictions to specific standards and/or have marketing authorization in certain non-U.S. regulatory jurisdictions. Authorization allows these products to be distributed to healthcare personnel only.

INLT Pandemic Supplies Webinar Thank You
A “thank you” from INLT.
INLT Pandemic Supplies Webinar Resources
Resources
FDA
- How to determine if your product is a medical device
- Overview of human drug requirements
- FDA Enforcement Policy for Face Masks and Respirators
- Emergency Use Authorizations
EPA